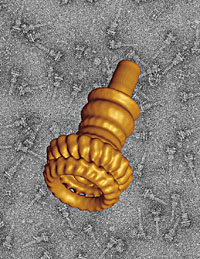
 | Pictured is the 3-D reconstruction of the base of the needle complex of the Salmonella bacterium; in the background, enhanced for contrast, the same structure is seen from many different angles. |
Even run-of-the-mill pictures are said to be worth a thousand words, but when describing stunning new images of the cell's molecular machinery created by structural biologists at Yale, Vinzenz M. Unger, associate professor of molecular biophysics and biochemistry, settles for a single pithy adjective: "mind-boggling."
In the next Dean's Workshop at the School of Medicine, Unger and four other medical school scientists will present the latest research using a cutting-edge technique that images molecules at extremely low temperatures. The workshop, "Cool Science: Cryoelectron Microscopy and Structural Biology at the Near-Atomic Scale," will be held Friday, June 10, 1:30-3:30 p.m. in the Anlyan Center auditorium, 300 Cedar St. The event is free and open to the public.
Scientists have long probed the nanoworld using electron microscopy (EM), but cryoelectron microscopy, or cryoEM, a technique that first came into its own during the 1990s, is particularly well-suited to biological research, say scientists.
The shower of electrons at the heart of EM incinerates proteins, so until recently biologists have had to coat the samples they wished to study with heavy metals. The resulting images showed a metal cast of the vaporized sample rather than the sample itself. In cryoEM, uncoated proteins are placed in water and plunged into liquid ethane, which cools the water at the rate of 100,000 degrees Centigrade per second, suspending the proteins in a protective ice-like solid that is utterly transparent.
The cryoEM technique lends itself to "single particle" imaging, in which the frozen sample contains dozens of copies of a structure of interest embedded at random orientations (a contrast-enhanced version is seen in the background of the accompanying image). Scientists feed tens of thousands of EM images of the structure seen from these myriad perspectives to powerful computers, which combine the information in the two-dimensional views to calculate the structure's three-dimensional form.
Jorge Galán, the Lucille P. Markey Professor of Microbial Pathogenesis, recently joined forces with Unger and Thomas Marlovits, a postdoctoral fellow in Unger's lab, on a cryoEM study that painted a vivid three-dimensional portrait of the base of the Salmonella bacterium's "needle complex," a syringe-like protein tube the food-borne bacterium uses to infect its host (see above).
As a measure of cryoEM's power, the base of the Salmonella syringe is 300 angstroms tall, while this page of newsprint is about 1 million angstroms thick.
-- By Peter Farley
T H I S
Workshop will explore technology's power to capture images of the molecular world W E E K ' S
W E E K ' S S T O R I E S
S T O R I E S![]()
 Team creates blood test for 'silent killer'
Team creates blood test for 'silent killer'![]()
![]()
 University marks 100 years of 'Pomp and Circumstance'
University marks 100 years of 'Pomp and Circumstance'![]()
![]()
 Yale scientist featured in new stamp series
Yale scientist featured in new stamp series![]()
![]()
 Twelve honored for strengthening town-gown ties
Twelve honored for strengthening town-gown ties![]()
![]()
 ENDOWED PROFESSORSHIPS
ENDOWED PROFESSORSHIPS Two appointed to Sterling chairs
Two appointed to Sterling chairs![]()
 R. Howard Bloch: French and Medieval Studies
R. Howard Bloch: French and Medieval Studies![]()
 Ian Shapiro: Political science and YCIAS
Ian Shapiro: Political science and YCIAS![]()
 Romano named Ruebhausen Professor
Romano named Ruebhausen Professor![]()
 Waxman is appointed to Flaherty chair
Waxman is appointed to Flaherty chair![]()
 Dr. State has been designated Harris Assistant Professor . . .
Dr. State has been designated Harris Assistant Professor . . .![]()
![]()
 Krauss named to second term at Silliman
Krauss named to second term at Silliman![]()
![]()
 Researchers discover virus' potential to target and kill deadly brain tumor
Researchers discover virus' potential to target and kill deadly brain tumor![]()
![]()
 Yale professors endow teaching and research fund in the history of science
Yale professors endow teaching and research fund in the history of science![]()
![]()
 Study shows, when it comes to fish genitalia, size has pros and cons
Study shows, when it comes to fish genitalia, size has pros and cons![]()
![]()
 Two Yale scientists honored with election to the NAS
Two Yale scientists honored with election to the NAS![]()
![]()
 Six Yale affiliates elected fellows of scholarly society
Six Yale affiliates elected fellows of scholarly society![]()
![]()
 Beijing conference explored Chinese constitutionalism
Beijing conference explored Chinese constitutionalism![]()
![]()
 New scholarship will help nurture future activist ministers
New scholarship will help nurture future activist ministers![]()
![]()
 Yale-IBM computer facility formally dedicated
Yale-IBM computer facility formally dedicated![]()
![]()
 REUNIONS
REUNIONS Alumni will gather for talks, tours and other special events
Alumni will gather for talks, tours and other special events![]()
 Library exhibit celebrates the accomplishments of the Class of '55
Library exhibit celebrates the accomplishments of the Class of '55![]()
 Medical school reunions feature talks on cutting-edge research
Medical school reunions feature talks on cutting-edge research![]()
![]()
 Yale launches research on lung cancer . . .
Yale launches research on lung cancer . . .![]()
![]()
 Workshop will explore technology's power to capture . . .
Workshop will explore technology's power to capture . . .![]()
![]()
 Show features artist's colorful depictions of 'Northern Shores'
Show features artist's colorful depictions of 'Northern Shores'![]()
![]()
 Glen Micalizio wins Beckman Young Investigator award . . .
Glen Micalizio wins Beckman Young Investigator award . . .![]()
![]()
 IN MEMORIAM
IN MEMORIAM Benjamin Mordecai: Drama school teacher and Broadway producer
Benjamin Mordecai: Drama school teacher and Broadway producer![]()
 Memorial service to honor South African historian Leonard Thompson
Memorial service to honor South African historian Leonard Thompson![]()
![]()
 Campus Notes
Campus Notes![]()
Bulletin Home |
| Visiting on Campus
Visiting on Campus |
| Calendar of Events
Calendar of Events |
| In the News
In the News![]()
Bulletin Board |
| Classified Ads
Classified Ads |
| Search Archives
Search Archives |
| Deadlines
Deadlines![]()
Bulletin Staff |
| Public Affairs
Public Affairs |
| News Releases
News Releases |
| E-Mail Us
E-Mail Us |
| Yale Home
Yale Home