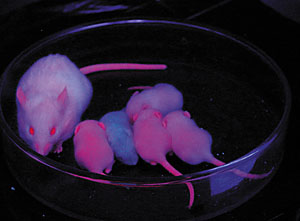
 | Yale researcher Tian Xu and his colleagues used a red fluorescent protein in specially bred mice to track the movement of a transposon called piggyBac. |
In a collaborative project, American and Chinese researchers developed a way to study the function of genes in mice and man by using a moveable genetic element from moths, according to a report in the journal Cell.
"We know how many genes are in the mammalian genome, but that does not tell us how they carry out their jobs," says senior author Tian Xu, professor and vice chair of genetics at the Yale School of Medicine and a Howard Hughes Medical Institute (HHMI) investigator. "We have found a way to systematically inactivate genes in the mouse genome so we can understand the functions of these genes."
After sequencing the human and mouse genomes, many scientists have shifted their attention to determining the function of all of those genes. The strategy is to mutate each gene, to observe the consequences, and investigate the molecular mechanisms. In the past two decades, only a small percentage of the genes shared by mice and humans have been analyzed in detail.
Genetic elements, called transposons, move from place to place in the DNA and allow material to be inserted or relocated. Bacteria swap antibiotic-resistance genes with transposons. Scientists have tailored this natural gene shuffling technique to insert genes and to mutate genes in fruit flies and simple organisms to learn the function of individual genes.
Transposons have proved to be valuable genetic tools for many organisms, but not for vertebrates and mammals. General application in mouse genetics was limited as they travel at low frequencies to limited locations, and had little capacity to carry DNA fragments. It took Xu's team six years to develop an efficient tool for genetic manipulations in vertebrates and mammals.
Xu and his colleagues at Fudan University in Shanghai finally chose a transposon called piggyBac that was originally identified in the cabbage looper moth. They discovered that it was stable and versatile in mouse and human cell lines, providing a new way to genetically manipulate mammalian cells. It also worked in mice even when it carried a couple of extra genes.
Xu's team made it easier to see the genes piggyBac associates with by adding a red fluorescent protein and an enzyme that changes the coat color of a white mouse to grey or black. The genes carried by the transposons have been stably inherited and expressed through five generations.
"The transposon acts as a genetic beacon, so researchers can easily track its location without having to sequence the entire genome," says Xu. In the scientists' experiments, piggyBac was incorporated into many chromosomes in human and mouse cells. PiggyBac can be removed from a mouse lineage by breeding with another mouse that has the enzyme to excise the transposon, explains Xu.
This technique is a powerful new tool for generating transgenic animals for vertebrates and mammals, and a potential new vehicle for human gene therapy, note the researchers. Although piggyBac inserts itself randomly into the DNA, it most often locates in genes, making it useful for mutating genes and thus, revealing gene functions.
This work has been hailed for showing scientists how transposons work in mammalian systems, while providing a tool for the systematic study of gene function throughout the human and mouse genomes.
In three months, the two graduate students who worked on the project generated mice mutating 75 different genes. Today, the researchers -- Xu; Min Han, an HHMI investigator at the University of Colorado, Boulder; Yuan Zhuang of Duke University; and their colleagues at Fudan University -- are in the process of scaling up piggyBac for the Mouse Functional Genome Project, which aims to mutate the majority of mouse genes at a state-of-the-art research facility in China to systematically understand the functions of the mammalian genes.
Xu expects the technique to be particularly useful for identifying genes and drug targets for diseases such as cancers and diabetes.
-- By Janet Rettig Emanuel
T H I S
Researchers create powerful tool
for decoding gene functions W E E K ' S
W E E K ' S S T O R I E S
S T O R I E S![]()
 Margaret Grey is named dean of School of Nursing
Margaret Grey is named dean of School of Nursing![]()
![]()
 Benson to step down as dean of School of Art after this year
Benson to step down as dean of School of Art after this year![]()
![]()
 Team discovers new planet in the outer solar system
Team discovers new planet in the outer solar system![]()
![]()
 Grant will fund center for study of nervous system
Grant will fund center for study of nervous system![]()
![]()
 Study: Alligator eggs show effect of oxygen on development
Study: Alligator eggs show effect of oxygen on development![]()
![]()
 Yale Librarian Prochaska appointed to a second term
Yale Librarian Prochaska appointed to a second term![]()
![]()
 New master's program prepares nurses for leadership roles
New master's program prepares nurses for leadership roles![]()
![]()
 Exhibit explores the 18th-century 'worlds' of Francis Wheatley
Exhibit explores the 18th-century 'worlds' of Francis Wheatley![]()
![]()
 Private portrait miniatures showcase the faces of public figures
Private portrait miniatures showcase the faces of public figures![]()
![]()
 Gallery hosting festive open house . . .
Gallery hosting festive open house . . .![]()
![]()
 Architecture gallery to feature traveling art show 'Ant Farm'
Architecture gallery to feature traveling art show 'Ant Farm'![]()
![]()
 Sterling Library launches new academic year with two exhibits
Sterling Library launches new academic year with two exhibits![]()
![]()
 Researchers create powerful tool for decoding gene functions
Researchers create powerful tool for decoding gene functions![]()
![]()
 Galapagos tortoises more diverse than once believed, say scientists
Galapagos tortoises more diverse than once believed, say scientists![]()
![]()
 Team identifies 'signatures' of protons in water
Team identifies 'signatures' of protons in water![]()
![]()
 'Canary Database' shows animals offer health warnings for humans
'Canary Database' shows animals offer health warnings for humans![]()
![]()
 Team digitally reconstructs long-extinct 'Lamp Shell'
Team digitally reconstructs long-extinct 'Lamp Shell'![]()
![]()
 'Gene trapping' reveals how flower development is controlled
'Gene trapping' reveals how flower development is controlled![]()
![]()
 Discovery may aid development of treatment for melanoma
Discovery may aid development of treatment for melanoma![]()
![]()
 Drinking alcohol may lower risk of non-Hodgkin's lymphoma
Drinking alcohol may lower risk of non-Hodgkin's lymphoma![]()
![]()
 Lyme disease prevention program launched in Connecticut
Lyme disease prevention program launched in Connecticut![]()
![]()
 For 35 students, summer was a time of service in New Haven
For 35 students, summer was a time of service in New Haven![]()
![]()
 IN MEMORIAM
IN MEMORIAM
 John Ostrom: He revolutionized field of paleontology
John Ostrom: He revolutionized field of paleontology![]()
 Robert Abelson: Psychologist was adviser to presidential candidates
Robert Abelson: Psychologist was adviser to presidential candidates![]()
 Greer Allen: University printer was inspiration to School of Art students
Greer Allen: University printer was inspiration to School of Art students![]()
![]()
 Yale Books in Brief
Yale Books in Brief![]()
![]()
 Campus Notes
Campus Notes![]()
Bulletin Home |
| Visiting on Campus
Visiting on Campus |
| Calendar of Events
Calendar of Events |
| In the News
In the News![]()
Bulletin Board |
| Classified Ads
Classified Ads |
| Search Archives
Search Archives |
| Deadlines
Deadlines![]()
Bulletin Staff |
| Public Affairs
Public Affairs |
| News Releases
News Releases |
| E-Mail Us
E-Mail Us |
| Yale Home
Yale Home